View solution When 100 mL of 04 M C H 3 C O O H are mixed with 100 mL of 02 M NaOH the H 3 O in 0. This means that if you have a N aCl solution with a concentration of 10 M the concentration of the N a ion will be 10 M and the concentration of the Cl ion will be 10 M.
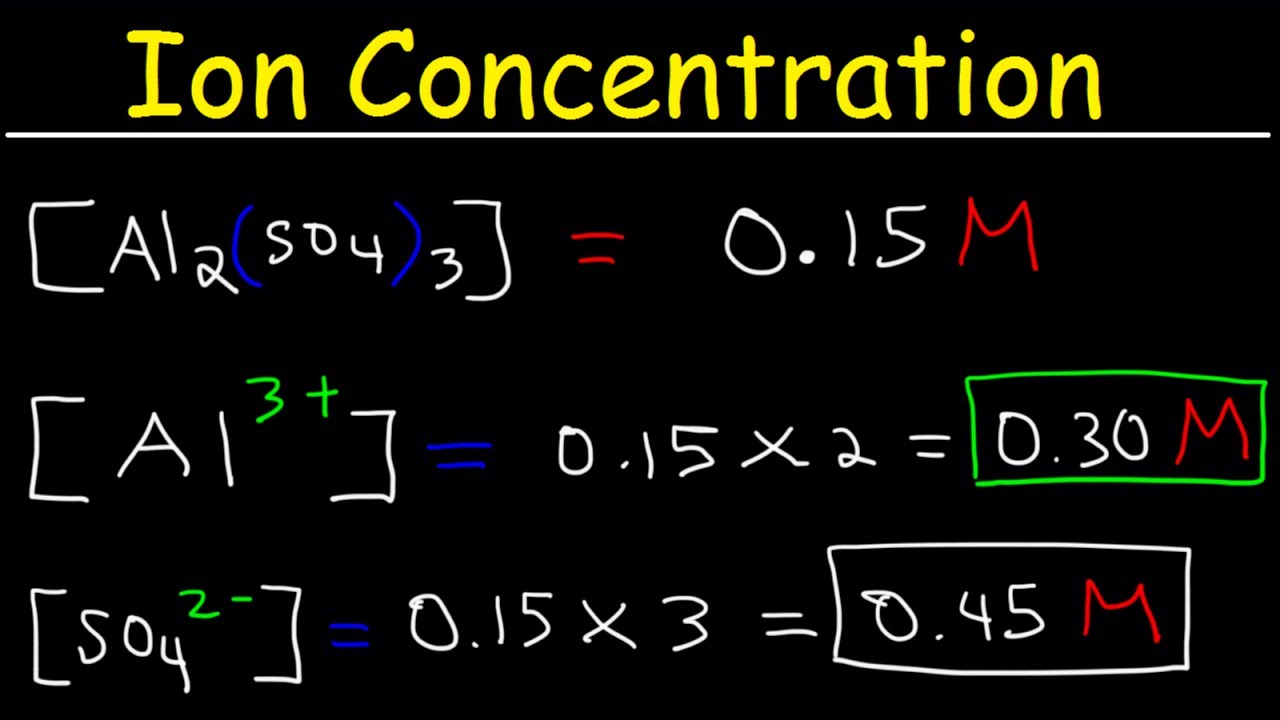
Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube
What is the total molar concentration of all the ions in 00200 M solutions of the following salts in water.

. From the given options the solution of 010M AlCl₃ is the one that has the highest concentration of chloride ions. The other options contain base K O H M g O H 2 and an organic species C 6 H 1 2 O 6 which will have low H 3 O ion concentration. 16 x 10-1 Calculate the number of moles of NaCl present in 750g of table salt.
Here we need to calculate the total ion concentration form the given ionic compounds and from the concentration of total ions we can determine compounds which has the highest total ions concentration. Which solution below has the highest concentration of hydronium ions. N aClaq N a aq Cl aq Sodium chloride dissociates into N a cations and Cl anions when dissolved in water.
The other options contain base K O H M g O H 2 and an organic species C 6 H 1 2 O 6 which will have low H 3 O ion concentration. Solution a The dissociation of the ionic solutes in the solution is represented by the equation describing the dissolution of Al 2 SO 4 3 s. 2 005 01 M.
From the given options an acid will have highest concentration of H 3 O ions. Notice that 1 mole of N aCl will produce 1 mole of N a and 1 mole of Cl. 1 010 M MgCl₂.
D 005 M CaCl₂ CaCl₂ Ca² 2Cl CaCl₂ dissociate into one mole of Ca² and two mole of Cl So concentration of chloride ions in 005 M CaCl₂ is. 10 A 005 M HBr B 01 M CH3OH C 01 M NaCl D 003 M Al2SO43 E 004. A solution of sodium dichromate Na2Cr2O7 is added to an acidic solution containing Fe2 ions.
5001 0050 M. Thus its concentration would be 5 times larger. So concentration of chloride ions in 010 M solution of MgCl₂ is 2 010 M 020 M.
The pH scale is based on a. 2 5 M H C O O H solution is found to have a p H of 2. This makes the total concentration of ions to be 0024 M.
A solution with a pH of 4 has twice the H of a solution with a pH of 2. 33001 results page 18 Chemistry. Which species NH4 or NH3 has the highest concentration in this buffered solution.
0 2 M H N O 3 will give highest concentration of H 3 O ions since it is an acid. The largest concentration would be 0010 M aluminum sulfate because it produces 5 ions when dissociated. 00200 M KCl 00200 M CuSO4 00200 M CaCl2.
The equation for the pH of a substance is pH logH where H is the concentration of hydrogen ions. Write the balanced net ionic equation for the following chemical reaction. Molarity is measured in number of moles of a.
To know which of the given solutions has the highest concentration of chloride ions we must evaluate each of them. Which of the following solutions has the highest total concentration of ions. A basic solution has a pH of 112.
10 Which of the following has the highest total concentration of ions. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. While the total concentration of K I is 002 M.
From the given options an acid will have highest concentration of H 3 O ions. If the concentration of Na in an aqueous solution of sodium sulfate in 32 x 10-1 molL how many moles of sodium sulfate must have been dissolved per liter to make the solution C. It dissociates in water and gives 3 ions one ion of barium and two ions of hydroxide with the concentration of 0008 M for each one.
In a saturated solution of AlOH3 the concentration of OH1- is 26 x 105. A buffered solution was created by mixing solutions of NH4Cl and NH3 at 25 C. 2 ions of Al³ and 3 ions of SO₄².
So the solution of barium hydroxide is a stronger electrolyte as it has a higher concentration of dissociated ions. We can see that in 1 mol of MgCl₂ we have 1 mol of Mg² and 2 moles of Cl ions so if the initial concentration. See the answer See the answer See the answer done loading.
025 M Ba3PO42 025 M Na2504 025 M ANO33 025 MKCI This problem has been solved. 0 2 M H N O 3 will give highest concentration of H 3 O ions since it is an acid.

Traces Of Ancient Life Tell Story Of Early Diversity In Marine Ecosystems Study School Of Medicine Analysis

Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube
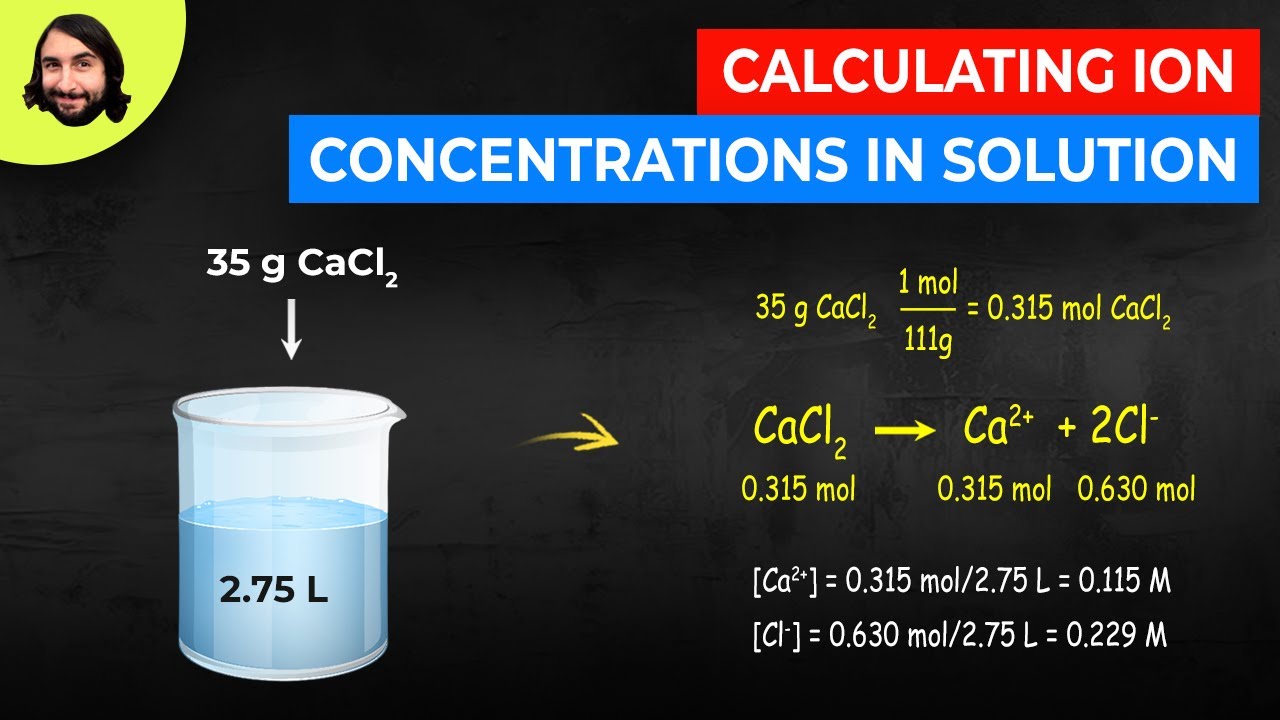
Calculating Ion Concentrations In Solution Youtube
Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube

Alchemik Colloidal On Instagram Colloidal Silver Explained Metallic And Ionic Silver Ions Atoms And Particles Ionic Silver Colloidal Silver Ionic

Pin By Homework Az On Chemistry Solutions Answers Concentration
16 6 Finding The H3o And Ph Of Strong And Weak Acid Solutions Chemistry Libretexts

Alchemik Colloidal On Instagram Colloidal Silver Explained Metallic And Ionic Silver Ions Atoms And Particles Ionic Silver Colloidal Silver Ionic

Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube
0 Comments